Researchers discovered that the enzyme OTULIN acts as a master regulator of tau and other genes linked to neurodegeneration. In human cell cultures, blocking OTULIN reduced tau levels and complete deletion stopped tau production without obvious neuronal harm. RNA sequencing showed dozens of additional genes — many tied to inflammation — were affected, indicating OTULIN influences neuronal stress pathways. These promising in vitro results require validation in animal and human studies, and any therapeutic approach will need careful control to avoid side effects.
OTULIN Identified as a Master Switch for Tau: A New Pathway in Alzheimer’s Research

Researchers at the University of New Mexico and the University of Tennessee report that the enzyme OTULIN, previously known mainly for regulating inflammation, also acts as a master regulator of genes linked to neurodegeneration — including production of the Alzheimer’s‑associated protein tau.
In a series of experiments on human tissue cultures, the team suppressed OTULIN activity and observed that tau protein levels fell. When the OTULIN gene was deleted entirely in those cultured neurons, tau production stopped. In these in vitro models, halting tau production this way did not appear to compromise neuronal health.
Neurons taken from a donor with Alzheimer’s disease contained higher levels of both OTULIN and tau compared with neurons grown from healthy donor stem cells, supporting a disease association.
Pathological tau is a central factor in brain aging and neurodegeneration, says molecular geneticist Karthikeyan Tangavelou of the University of New Mexico. He adds that targeting OTULIN to stop tau synthesis could potentially help prevent aspects of brain aging, though this remains to be tested.
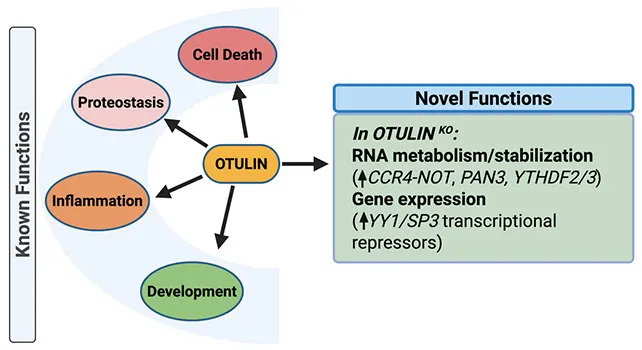
The researchers also performed RNA sequencing to understand broader effects of OTULIN loss. Deletion of OTULIN altered the activity of dozens of other genes, many of which are involved in inflammatory pathways and cellular stress responses. This suggests OTULIN may influence neuronal stress and the brain s wear-and-tear when normal clearance and regulatory systems fail.

Implications and Cautions
Although these findings point to OTULIN as a promising new research target, the authors emphasize caution. Both OTULIN and tau have important roles in normal physiology, and interventions that reduce OTULIN activity could have unintended effects elsewhere in the body or brain. The current results come from cell-culture experiments and must be validated in animal models and further human studies before any clinical application.
One known function of OTULIN is to help regulate cellular clearance mechanisms that remove waste, including aggregated or excess proteins such as tau. When OTULIN function is impaired, these clearance systems can fail and protein build-up may follow — a possible contributing factor to neurodegeneration.
The study has been published in the journal Genomic Psychiatry. The authors describe OTULIN as a new avenue for research into Alzheimer s disease and brain aging, while stressing that therapeutic targeting will require precise, carefully controlled approaches.
Help us improve.