Researchers at the University of Utah found that the gut bacterium Turicibacter reduces weight gain and improves metabolic markers in mice fed a high‑fat diet by producing fatty acids that counter harmful ceramides. High dietary fat — especially palmitate — suppresses Turicibacter growth, so mice given oral supplements five days a week showed smaller weight gains, lower fasting glucose and improved lipid profiles. The results are promising but preliminary; further studies are needed to determine whether the findings translate to humans.
Single Gut Bacterium, Turicibacter, Blunts Weight Gain in Mice on High‑Fat Diet — A Step Toward Microbe-Based Therapies

Researchers at the University of Utah report that a single gut bacterium, Turicibacter, can markedly reduce weight gain and improve metabolic markers in mice eating a high‑fat diet. The new study, published in Cell Metabolism, suggests molecules produced by this microbe alter fat handling in the small intestine and restrain harmful lipids linked to metabolic disease.
Key Findings
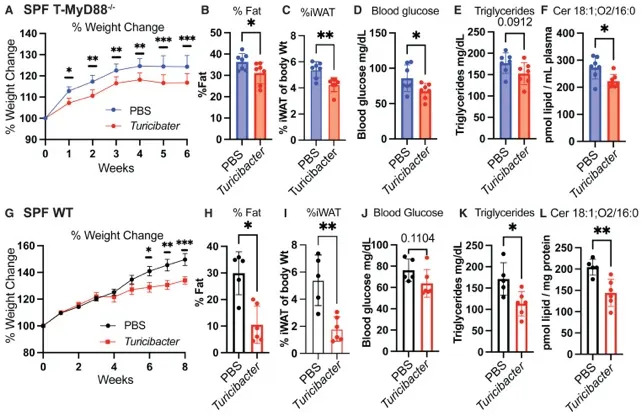
The team, led by microbiologists including June Round and first author Kendra Klag, found that mice given an oral Turicibacter supplement five days a week gained less weight, had lower fasting glucose, reduced body fat and improved lipid profiles despite continued high‑fat feeding. The bacterium produces a suite of fatty acids that appear to counteract the rise of ceramides — lipid molecules that increase on high‑fat diets and are associated with insulin resistance, type 2 diabetes and cardiovascular risk.
How It Works — And Its Limits
Laboratory experiments showed that palmitate, a common saturated fat abundant in many high‑fat diets, halted growth of Turicibacter but did not kill it; the bacteria resumed growth when palmitate was removed. That pattern suggests that although Turicibacter can protect against some metabolic harms, very high dietary fat undermines its abundance. Because high‑fat feeding both promotes ceramide accumulation and suppresses protective microbes like Turicibacter, maintaining beneficial levels may require supplementation or other interventions.

Context and Caution
This work adds to a growing body of evidence linking the gut microbiome to metabolic health. Previous studies showed that transferring microbes from obese animals can transfer weight‑gain tendencies to lean, germ‑free animals, while other experiments found that removing gut microbes can sometimes protect against diet‑induced weight gain — underscoring that specific microbial communities, not simply presence or absence of bacteria, shape metabolic outcomes.
"I didn't think one microbe would have such a dramatic effect — I thought it would be a mix of three or four," said June Round. Kendra Klag added that with further study, microbes could be developed into tailored therapies: "We will be able to make microbes into medicine and find bacteria that are safe to create a consortium of different bugs that people with different diseases might be lacking."
Implications
While promising, these results are preclinical and were observed in mice. Translation to humans will require careful testing to confirm safety, dosing and effectiveness. The findings point toward two complementary paths: identifying microbial species with protective metabolic effects and isolating their bioactive molecules for therapeutic development. Unlike current GLP‑1 drugs such as Ozempic, microbe‑based approaches could, in principle, be personalized to a person’s microbiome and might avoid some drug side effects — but that remains to be demonstrated.
Publication: Cell Metabolism.
Help us improve.


































