Researchers at the University of Minnesota show that degrading bacterial AHL signals in lab-grown dental plaque can shift the oral microbiome toward healthier species. Enzymes that block quorum sensing reduced growth of bacteria associated with gum disease, with biofilms proving more responsive than planktonic bacteria. Results come from simplified laboratory models and have not yet demonstrated direct clinical effects on cavities or periodontal disease.
Silencing Bacterial 'Chatter' Could Help Protect Teeth, Study Finds

New laboratory research from the University of Minnesota suggests that interrupting chemical communication among mouth microbes can encourage beneficial species and may reduce factors that lead to tooth decay and gum disease.
How Bacteria 'Talk'
Bacteria in the mouth use a chemical signaling system called quorum sensing to coordinate gene expression and population behavior. One well studied class of signals is N‑Acyl homoserine lactones (AHLs), which some oral microbes produce and detect to influence which species thrive in dental plaque.
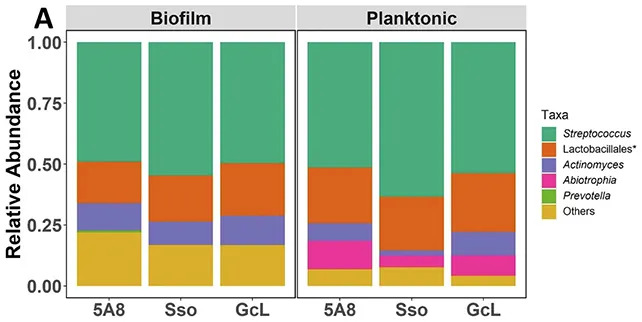
What The Researchers Did
The team grew simplified bacterial communities in the lab that model human dental plaque and tested enzymes that degrade or inhibit AHL signals. By disrupting these chemical messages, they were able to shift community composition so that health-associated bacteria increased while species linked with periodontal disease declined.
Mikael Elias, biochemist — By disrupting the chemical signals bacteria use to communicate, one could manipulate the plaque community to remain or return to its health-associated stage.
Key Findings
Notable results include the following:

- Biofilm communities, which grow on tooth and gum surfaces, were more responsive to AHL disruption than free-floating bacterial populations.
- Communities cultured under oxygen-poor conditions, which mimic deep plaque niches, responded differently from those grown in oxygen-rich conditions.
- Even when anaerobic bacteria do not produce AHLs themselves, they can still sense AHL signals made by other microbes, suggesting cross-talk across different oral niches.
Rakesh Sikdar, biochemist — Quorum sensing may play very different roles above and below the gumline, which has major implications for how we approach treatment of periodontal diseases.
Limitations And Outlook
The study used simplified lab models of dental plaque and did not measure direct clinical outcomes such as reductions in cavities or periodontal disease severity. Additional work is needed to confirm these mechanisms in real human mouths and to test safety and efficacy in living organisms.
Nonetheless, the findings offer a promising alternative strategy to traditional antimicrobial approaches: instead of trying to eliminate oral microbes, researchers may be able to shift communities toward a healthier balance by targeting their communication pathways. Because oral health is linked to systemic conditions affecting the brain and heart, the authors suggest similar approaches might one day help manage infections in other body sites.
The research is published in NPJ Biofilms and Microbiomes.
Help us improve.


































