Ben‑Gurion University researchers report a new class of latent monomers that remain liquid for weeks and polymerize only when triggered. Using norbornadiene/quadricyclane chemistry, a standard ROMP catalyst and gold nanoparticles as microscopic heaters, the system stores monomers in an inert state and reactivates them with mild heating (e.g., near‑IR‑heated gold). The approach enables safer storage, selective curing for 3D printing and coatings, and the creation of materials with regions of differing mechanical properties.
Ben‑Gurion Team Develops Light‑Activated Liquid Plastic That Cures On Demand

Researchers at Ben‑Gurion University of the Negev have engineered a “smart” liquid formulation whose monomer building blocks contain an integrated on/off switch, removing the need for fragile or costly dormant catalyst systems and enabling safer, more flexible manufacturing.
Described last month in Nature Chemistry, the work introduces a new family of latent monomers: stable liquid precursors that can remain dormant for weeks and be triggered to polymerize only when needed. The approach promises to simplify industrial curing, add flexibility to 3D printing and coatings, and reduce material waste and energy use.
How It Works
The formulations combine three core components: (1) molecular building blocks derived from norbornadiene, (2) a conventional ROMP (ring‑opening metathesis polymerization) catalyst, and (3) minute gold nanoparticles that act as localized heaters when illuminated by near‑infrared light.
Key to the design is a reversible chemical switch based on norbornadiene/quadricyclane isomerization. The monomers can be converted into an inert “sleeping” form, quadricyclane, by photoisomerization; in this state they do not undergo polymerization and remain fluid. When polymerization is desired, gentle heating—for example, via the gold nanoparticles illuminated with near‑IR—reverts quadricyclane to the reactive norbornadiene, allowing the ROMP catalyst to build long polymer chains on demand.
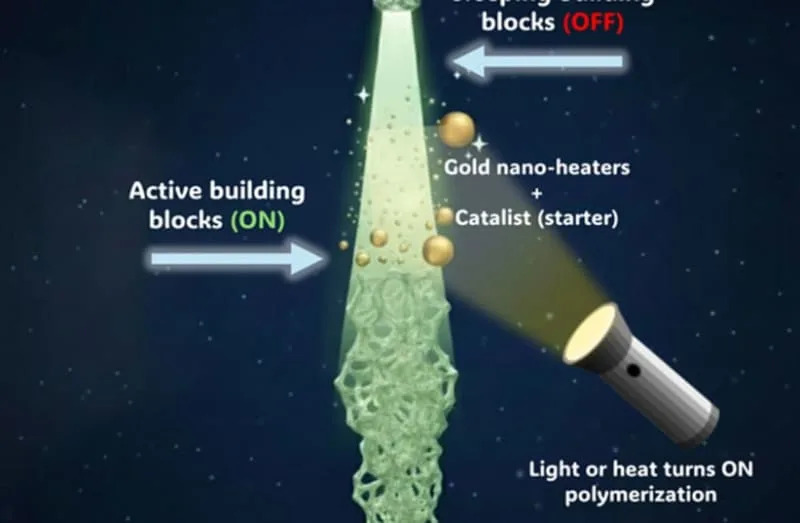
Practical Advantages
Because the material remains inert until triggered, manufacturers could ship, store, and use ready‑to‑apply liquid formulations without the risk of premature curing. Parts can be filled, coated, or printed first and then selectively hardened by applying patterned light or localized heating, reducing waste and avoiding the need to heat or remix large volumes.
The team also demonstrated a route to materials with spatially varied properties by blending always‑active monomers with dormant ones. This enables creation of polymers whose chains have two distinct segments—allowing a single part to start out soft and then be locally converted into a tougher, more durable region in the same processing sequence.
“Instead of a ‘sleeping’ catalyst, we created ‘sleeping’ building blocks of the material itself,” said Prof. Yossi Weizmann of Ben‑Gurion University. “The mixture can sit quietly on the shelf for weeks and will snap together into a solid only when you shine light on it or warm it up.”
Project contributors include PhD student Nir Lemcoff (lead author), Ronny Niv and Keren Iudanov from the Lemcoff group, and Gil Gordon, Aritra Biswas, Uri Ben‑Nun and Ofir Shelonchik from collaborating labs. The team emphasizes that the embedded switch strategy could inspire new approaches across polymer science and manufacturing.
Potential impact: On‑demand curing could make industrial production, additive manufacturing and repair operations safer, simpler and more energy efficient by eliminating delicate dormant catalysts and enabling localized, low‑energy activation.
Help us improve.