The Weizmann Institute team has uncovered how compensatory proliferation — a rapid tissue‑repair response first seen in fly larvae — is executed at the molecular level. They identified two cooperating survivor cell types: DARE cells, which activate the initiator caspase Dronc but survive and proliferate, and NARE cells, which are recruited to assist and regulate repair. Descendants of DARE cells become about seven times more resistant to cell death, and the motor protein Myo1D helps protect these survivors. The results, reported in Nature Communications, may explain how recurrent tumours gain resistance and point to ways to either boost healing or limit cancer relapse.
How Tissues 'Resurrect' Cells After Damage — Fly Study Solves 50‑Year Mystery

When tissue suffers severe injury, surviving cells can mount a focused burst of repair known as compensatory proliferation. Nearly 50 years after the response was first observed in fruit‑fly larvae, researchers at the Weizmann Institute of Science have identified the molecular mechanism that drives it.
The team revisited the classic experiment that first revealed compensatory proliferation: high‑dose irradiation of Drosophila melanogaster larvae. Using a delayed molecular sensor, they tracked cells in which an initiator caspase had been activated yet that nevertheless survived the radiation insult. That approach uncovered two distinct types of survivor cells that cooperate to regenerate damaged tissue.
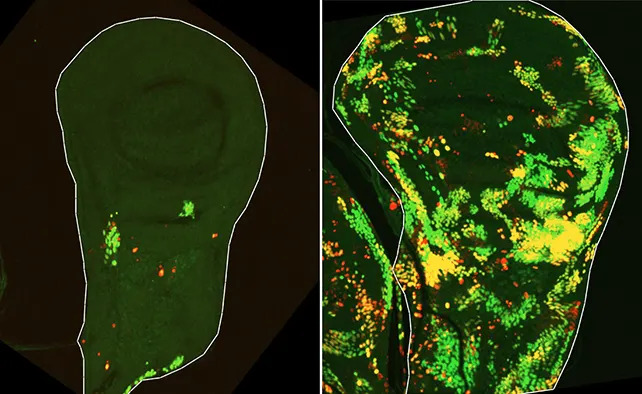
Two Survivor Populations: DARE and NARE
The first group, called Dronc‑Activating (DARE) cells, transiently activate the initiator caspase Dronc. Instead of dying, these cells resist apoptosis and rapidly proliferate to replace lost tissue. The second population, termed NARE cells, do not show initiator caspase activation but are recruited by DARE cells to assist and help regulate the repair so regeneration does not overshoot.
“We set out to identify cells that push the self‑destruct button but survive anyway,” said Tslil Braun, first author and molecular geneticist at the Weizmann Institute.
Resistance After Repair And A Protective Motor Protein
Importantly, descendants of the DARE cells and the tissue they generate become markedly more resistant to subsequent damage. After a second radiation exposure, the lineage derived from DARE cells was approximately seven times more resistant to cell death than cells in the original tissue — a behavior reminiscent of therapy‑resistant recurrent tumors.

The team also identified the molecular motor protein Myo1D as a factor that helps protect DARE cells from dying. Because Myo1D has been implicated in cancer biology, the researchers note that tumours might exploit similar protective machinery to survive treatment.
“The descendants of DARE cells were found to be exceptionally resistant — seven times more resistant to cell death than cells in the original tissue,” said Eli Arama, molecular geneticist at the Weizmann Institute.
Implications And Caution
These findings, published in Nature Communications, illuminate how compensatory proliferation is orchestrated at the molecular level. Understanding this mechanism could inform two opposite therapeutic goals: enhancing beneficial regeneration after injury, or blocking the same pathway to prevent cancer recurrence.
However, the experiments were performed in fruit flies, and the authors emphasize that further work is needed to confirm whether the same mechanisms operate in human tissues.
Help us improve.


































