Case Western Reserve researchers report a three‑year study showing that alpha‑synuclein binds and impairs the mitochondrial enzyme ClpP, linking protein aggregation to mitochondrial dysfunction in Parkinson’s disease. They designed a decoy peptide, CS2, that blocks this interaction and, in lab tests on human tissue, mice, and cultured neurons, reduced inflammation and partially restored motor and cognitive function. Human clinical trials could begin in roughly five years, pending extensive safety testing. The work highlights a promising mitochondria‑targeted therapeutic approach while underscoring the complexity of Parkinson’s.
Researchers Reveal How Alpha‑Synuclein Damages Mitochondria — New Peptide CS2 Restores Neuronal Function in Parkinson’s Models

Researchers at Case Western Reserve University School of Medicine say they have closed a long-standing gap in our understanding of how toxic protein buildup in Parkinson’s disease leads to the loss of essential brain cells. After three years of investigation, the team reports a direct molecular link between alpha‑synuclein and mitochondrial dysfunction — two features long associated with Parkinson’s but not previously connected at the mechanistic level.
In laboratory experiments the scientists observed that alpha‑synuclein binds to a mitochondrial enzyme called ClpP, which is involved in protein quality control and waste removal inside mitochondria. The study shows that this binding interferes with ClpP’s activity, impairing mitochondrial function and producing downstream effects common in Parkinson’s patients, including reduced dopamine production and cellular energy deficits.
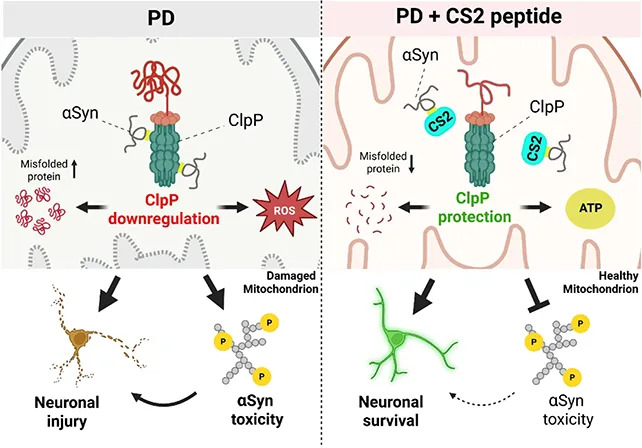
“We’ve uncovered a harmful interaction between proteins that damages the brain’s cellular powerhouses, called mitochondria,” says neuroscientist Xin Qi. “More importantly, we’ve developed a targeted approach that can block this interaction and restore healthy brain cell function.”
Building on that finding, the team designed a short peptide, named CS2, that acts as a decoy for alpha‑synuclein. By preferentially binding alpha‑synuclein, CS2 diverts it away from ClpP and the mitochondria, preventing the damaging interaction. In tests on human brain tissue, mouse models, and neurons grown in the lab, CS2 reduced markers of brain inflammation and partially restored motor and cognitive function in animals.
“This represents a fundamentally new approach to treating Parkinson’s disease,” says neurophysiologist Di Hu. “Instead of just treating the symptoms, we’re targeting one of the root causes of the disease itself.”
The researchers estimate it may be about five years before human clinical trials begin to evaluate CS2’s safety and efficacy. They emphasize caution: manipulating protein interactions and mitochondrial processes can have unintended effects, so rigorous preclinical testing is required.

Although the findings are an important advance — identifying a specific molecular fault and demonstrating a potential way to correct it — Parkinson’s is a complex disease in which causes and consequences are often intertwined. Experts expect that multiple therapeutic strategies will be needed to halt or reverse the disease, and mitochondria‑targeted therapies like CS2 could become one important component of a broader treatment approach.
“One day we hope to develop mitochondria‑targeted therapies that will enable people to regain normal function and quality of life,” Qi adds. “We aim to transform Parkinson’s from a crippling, progressive condition into a manageable — or resolved — one.”
The study is published in the journal Molecular Neurodegeneration.
Help us improve.




























